Two Chinese pharmaceutical firms kicked off two-month clinical tests on the country's A/H1N1 influenza vaccines Wednesday.
More than 2,000 volunteers recruited in Taizhou in eastern Jiangsu Province took part in the trials carried out by Hualan Biological Engineering Inc., said Fan Bei, deputy general manager of the company.
The tests came exactly one month after the vaccines were produced by the Henan-based company, and submitted for laboratory test in June.
|
|
|
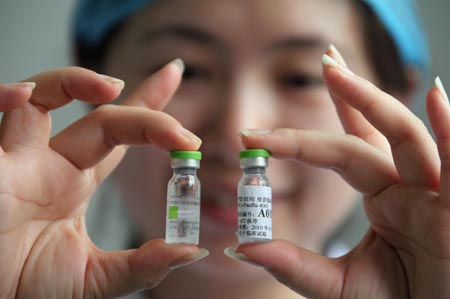
A medical staff displays A/H1N1 influenza vaccines in Beijing, capital of China, July 22, 2009. Sinovac Biotech Co. Ltd. began clinical test on A/H1N1 influenza vaccines Wednesday in Beijing. The volunteers will inoculate the first batch of A/H1N1 influenza vaccines in a week and the research will be completed in September. [Xinhua] |
?Hualan was among the first in China to receive the seed virus of the A/H1N1 flu on June 3, and produced the country's first batch of A/H1N1 vaccines, 90,000 doses in all, on June 22.
The clinical trials, supervised by the Chinese Center for Disease Control and Prevention, were aimed at specifying the dosage and the immunity procedures of the vaccines, said Fan.
The volunteers, divided into five age groups, would receive the vaccines in turns on Wednesday, Friday and Saturday. A second round of inoculation is due 21 days after the initial vaccination.
During the two-month clinical test, the volunteers would have antibody tests for four times, Fan said. Follow-up tests would continue till six months after their initial vaccination.
Previous reports said the vaccines were expected to hit the market in September should they pass both laboratory and clinical tests, and the Hualan Biological Engineering Inc. would be able to make 600,000 doses a day.
In the meantime, the Chinese Center for Disease Control and Prevention and its Beijing bureau also on Wednesday began clinical tests on the vaccines produced by the Sinovac Biotech Ltd., or the Beijing Kexing Bioproducts, in Beijing.
A total of 1,600 participants took part in the test, and would all receive the vaccines this week, according to Yin Weidong, general manager of Kexing.
Participants aged between 3 and 60 would receive another round of vaccination 21 days after their initial vaccination, same as the participants in the Hualan test. But participants aged over 60 would only receive first one round of inoculation, Yin said.
|
|
|
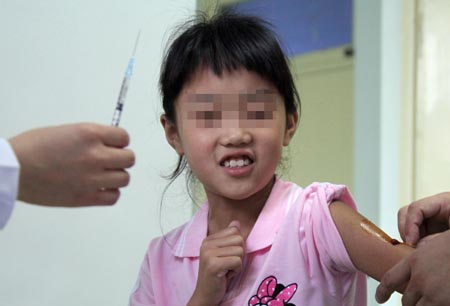
A medical staff injects A/H1N1 influenza vaccine to a volunteer in Beijing, capital of China, July 22, 2009. [Xinhua] |
?The clinical test would be completed by mid September, he said.
As of 6 p.m. of July 22, China had reported 1,772 cases of the A/H1N1 influenza on the mainland, of whom 1,454 had recovered and been discharged from hospital, the Health Ministry said Wednesday.
No deaths or critical cases of the virus have been reported on the Chinese mainland, but a patient in Hangzhou of eastern Zhejiang Province was reportedly electrocuted in a hospital bathroom on July 1.
Across the world, more than 700 people have died from the influenza since April, the World Health Organization (WHO) said on Tuesday.
Countries are racing for a A/H1N1 vaccine, as Marie-Paule Kieny, director of WHO's Initiative for Vaccine Research, said at a WHO expert group last week that the influenza was "unstoppable," and that "all countries need to have access to vaccines."
Australian pharmaceutical company CSL also began a clinical test on its A/H1N1 flu vaccine on Wednesday, saying that it could have an A/H1N1 vaccine ready by September if its clinical trials go well.
(Xinhua News Agency July 23, 2009)