For many years the Chinese government has been continuously improving its drug safety supervision system, its drug safety technical supporting system and related laws and regulations.
In 1998 the State Drug Administration was established, and the State Food and Drug Administration (SFDA) was founded on the basis of the former in 2003. The SFDA is in charge of administrative supervision and technical supervision over the research, production, distribution and use of drugs (including Chinese crude drugs, prepared slices of Chinese crude drugs, traditional Chinese medicine preparations, chemical drug substances and their preparations, antibiotics, biochemical drugs, radioactive pharmaceuticals, serum, vaccines, blood products and diagnostic agents) and medical devices. China has established drug regulatory departments under the unified leadership of the central government, with vertical management below the provincial level. By the end of 2007, there had been 2,692 drug regulatory departments in China, including 31 at the provincial level, 339 at the municipal (prefecture) level and 2,321 at the county (county-level city) level (including districts and counties of municipalities directly under the central government); with more than 1,000 drug technical supervision organizations with 64,000 personnel in China. In the vast rural areas, drug safety coordinators and information specialists had been employed to ensure drug safety and promote the building of a drug safety supervision network. By the end of 2007, there had been more than 97,000 drug safety coordinators and more than 514,000 information specialists in rural areas. A total of 578,000 stations of rural drug regulatory network had been established.
The state has been steadily increasing its financial investment into drug safety supervision, with the emphasis on improving the drug safety testing level and ability, and providing technical support for the drug safety supervision work. National-level drug technical supervision organizations mainly include the National Institute for the Control of Pharmaceutical and Biological Products, Chinese Pharmacopoeia Commission, Center for Drug Evaluation, Center for Drug Certification, National Committee on the Assessment of the Protected Traditional Chinese Medicinal Products, Center for Drug Re-evaluation, National Center for ADR Monitoring and Center for Medical Devices Evaluation. These organizations, all affiliated to SFDA, take the responsibility of routine testing, testing methodology research, conservation of breeds of experimental animals, standardization research, technical evaluation of registration applications and ADR monitoring. In addition, there are 19 national port institutes for drug control taking the responsibility of registration testing and port testing of import drugs; 33 provincial-level institutes for drug control in charge of sample testing, retesting, consigned drug testing, new drug registration testing and state-planned sample testing within their respective jurisdictions, as well as the drafting of national drug standards; 325 municipal (prefecture)-level institutes for drug control in charge of sample testing and consigned testing of drugs within their respective jurisdictions.
With respect to the supervision of medical devices, a technical testing system at both the national and provincial levels has taken initial shape. There are ten national-level centers for medical device quality testing, which take the responsibility of registration testing of domestic Class III medical devices and import medical devices, as well as sample testing for the quality of Chinese medical devices. There are 30 provincial-level medical device testing organizations, responsible for sample testing of medical devices within their respective jurisdictions, as well as the registration testing of some types of medical devices. There are nine medical device testing organizations established by specialized universities and research institutes. In addition, there are 22 medical device standardization technical committees for different fields of specialization.
China attaches great importance to the building of a legal system for drug safety supervision. In 1984 the Drug Administration Law of the People's Republic of China was adopted by the Standing Committee of the National People's Congress (NPC). For the first time, the research, production, selling and use of drugs were covered by legal stipulations, and the legal responsibility for the production and sale of counterfeit and inferior drugs was defined. This symbolizes that China's drug administration work is now managed in accordance with the law. This Law was revised in 2001 to unify drug standards and abolish regional standards; heighten the legal responsibility for the production and sale of counterfeit and inferior drugs; and define Good Manufacturing Practice (GMP) and Good Supply Practice (GSP) as legal requirements. The Drug Administration Law of the People's Republic of China and other relevant laws and regulations provide a legal guarantee for drug administration ensuring the drug quality and protecting people's legal rights of drug use.
So far, the State Council has promulgated 17 administrative regulations concerning drugs, including Special Rules on Strengthening the Supervision and Management of the Safety of Food and Other Products, Regulations for the Implementation of the Drug Administration Law of the People's Republic of China, Regulations for the Control of Narcotic Drugs and Psychotropic Drugs, Measures for the Control of Radioactive Drugs, Regulations for the Control of Blood Products, Regulations for the Administration of Distribution of Vaccines and Vaccination, Anti-doping Regulations, Regulations for the Administration of Precursor Chemicals and Regulations for the Protection of Traditional Chinese Medicines.
According to the Drug Administration Law of the People's Republic of China, the national drug regulatory department has formulated 29 provisions, including Provisions for Drug Recall, Provisions for Drug Registration, Good Laboratory Practice (GLP), Good Clinical Practice (GCP), Good Manufacturing Practice (GMP), Provisions for the Drug Distribution Licenses, and Good Supply Practice (GSP). The state drug regulatory department has also jointly promulgated provisions with the health, industry and commerce, and customs authorities, including Provisions for ADR Reporting and Monitoring, Standards for the Examination and Publicizing of Drug Advertisements, Provisions for the Examination of Drug Advertisements, Provisions for the Import Drugs, and Provisions for the Import and Export of Protein Assimilation Preparations and Peptide Hormones (Provisional).
The Chinese government attaches great importance to the formulation of administrative regulations concerning medical devices. In 2000 the State Council promulgated the Regulations for the Supervision and Administration of Medical Devices. The state drug regulatory department has drawn up ten related regulations, including the Provisions for Medical Device Registration, Rules for Medical Device Classification, Provisions for Medical Device Standards, Provisions for Clinical Trials of Medical Devices, Good Manufacturing Practice (GMP) for Medical Devices, Provisions for Evaluation of the Quality System of Medical Device Manufacturers, and Provisions for Indications, Labels and Packaging Marks of Medical Devices. The state drug regulatory department has also promulgated, together with the industry and commerce departments, the Standards for the Examination of Medical Device Advertisements and Provisions for the Examination of Medical Device Advertisements.
Moreover, China has established a national drug standard system based on the Chinese Pharmacopoeia and standards set by the State Food and Drug Administration. Drug standards and quality standards for some medical devices are compulsorily applied nationwide. There are more than 15,000 national drug standards in China; and 686 medical device standards, of which 155 are national standards and 531 are industrial standards.
Figure 3 Varieties of Drugs Recorded in the Chinese Pharmacopoeia
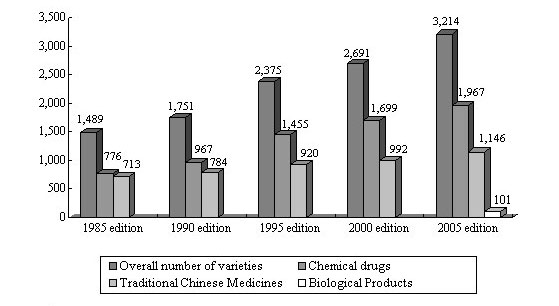
As early as in 1953, the Chinese government published the Chinese Pharmacopoeia. It has so far gone through eight editions, with the 2005 edition being the current edition. The 2005 edition adopted considerably more drug varieties than before, with the application of more modern analysis technologies, and more emphasis is placed on drug safety parameters. Additions and revisions are made to the general rules, analysis and testing methods and guidelines in respect of preparations. There are 1,146 monographs in Volume I, including Chinese crude drugs, prepared slices of Chinese crude drugs, herbal oil, fats and extracts, and compound and single prescriptions; Volume II admits 1,967 chemical drugs, antibiotics, biochemicals, radioactive pharmaceuticals and pharmaceutical excipients; Volume III admits 101 biological products.
China attaches great importance to raising and unifying national drug standards, and encouraging enterprises to formulate and apply registration standards higher than the national standards. The relevant departments of the Chinese government are unifying and raising the current national drug standards by stages and in batches, so that the testing technology of national drug standards will eventually reach the international advanced level.